Chapter 8: Dilutions
Sometimes you will need to change the concentration of solution by changing the amount of solvent. This is often done when working in the laboratory or when dispensing drugs. Dilution is where additional solvent is added to a solution to decrease the concentration of the solute. It is important to remember that with dilution the amount of solute remains constant. It is only the concentration that changes.
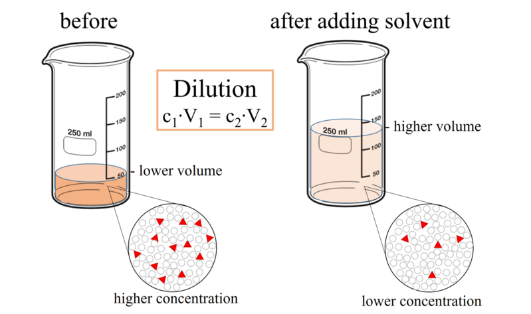
The ability to accurately dilute is important as it allows many ‘working’ solutions of different concentrations to be made from a single concentrated stock solution. As the amount of solute remains the same following dilution the ratio between the concentration and volume remains constant before and after dilution. This can be exploited to work out a final concentration following dilution or the volume of additional solvent to add to achieve a target concentration.
You may come across the term ‘dilution factor’. This refers to the change in volume of the solvent. For example, if you add 90 ml of water to 10 ml of an aqueous solution, you have increased the volume from 10 ml to 100 ml. This represents a 10-fold dilution (interchangeably referred to as a 1 in 10 dilution, 1/10 dilution or 10x dilution). Some concentrated stock solutions will be labelled with the suggested working dilution. For example, a commercial buffer may be labelled as 5x, which means it needs to be diluted five-fold for use. Often the dilution factor is simply stated as a number.To calculate the dilution factor, you need to determine the ratio of the volume of the initial (concentrated) solution (V1) to the volume of the final (dilute) solution (V2).
So, the dilution factor = V2 ÷ V1
Note that since this is a ratio it is important that both volumes are in the same units.

