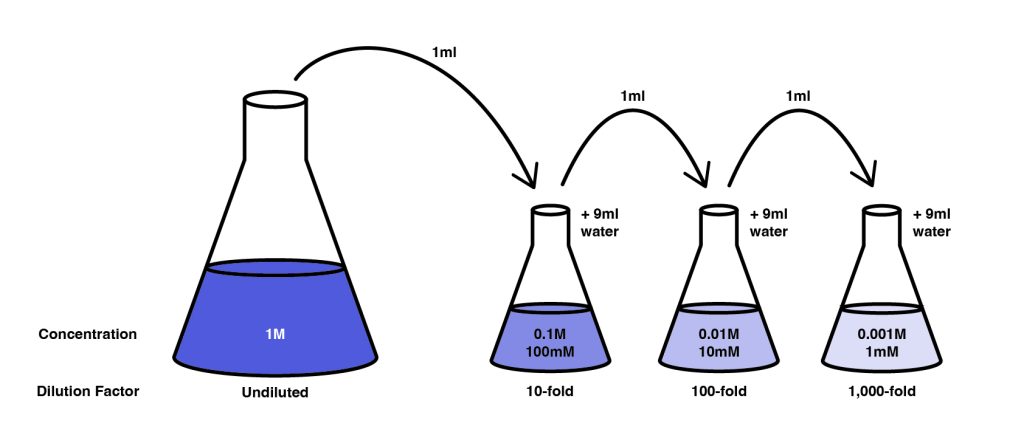
8.1 Serial dilutions
To make large dilutions, multiple serial dilutions of the initial stock are often made. In this case you can determine the final dilution factor by multiplying the factors of each dilution. The final concentration can be determined by dividing the initial concentration by the dilution factor.
Concentration (verb)
As we saw in the previous chapter, concentration is the reverse process, where either solvent is removed or solute is added to increase the concentration of the solute.
How to concentrate solutions
There are several methods to reduce the volume of solvent. One of the simplest ways is by applying heat to evaporate some of the solvent. However, this method is not always appropriate. For example, proteins would be denatured by this process.
Other ways include removing the solvent by freeze drying (lyophilisation) or by precipitating the solute (in the case of proteins or nucleic acids), allowing the solvent to be removed before redissolving in a smaller volume.
A common way to concentrate macromolecules is to use a semi-permeable membrane which allows solvent molecules to pass through but not the larger solute molecules. Such a semi-permeable barrier can be incorporated into a centrifuge tube where centrifugal force pushes the solution against the barrier, concentrating protein in the remaining solution.
Or the solution can be dialysed (placed in a sac made from the semi-permeable membrane) and the sac put into a solution of high concentration where again the solute molecules cannot pass through the membrane. In this case water will gradually move from the sac to equilibrate with the surrounding solution by a process called osmosis, decreasing the volume, and concentrating the solution in the sac.

