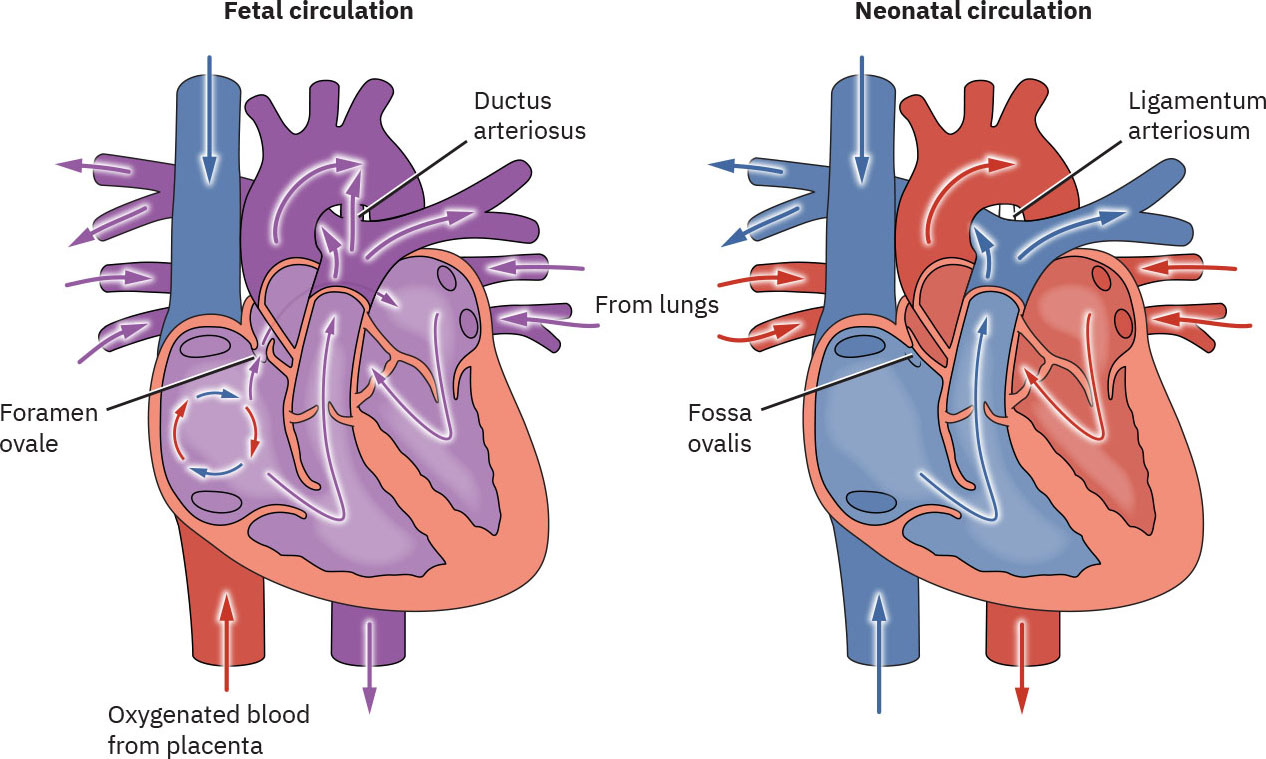
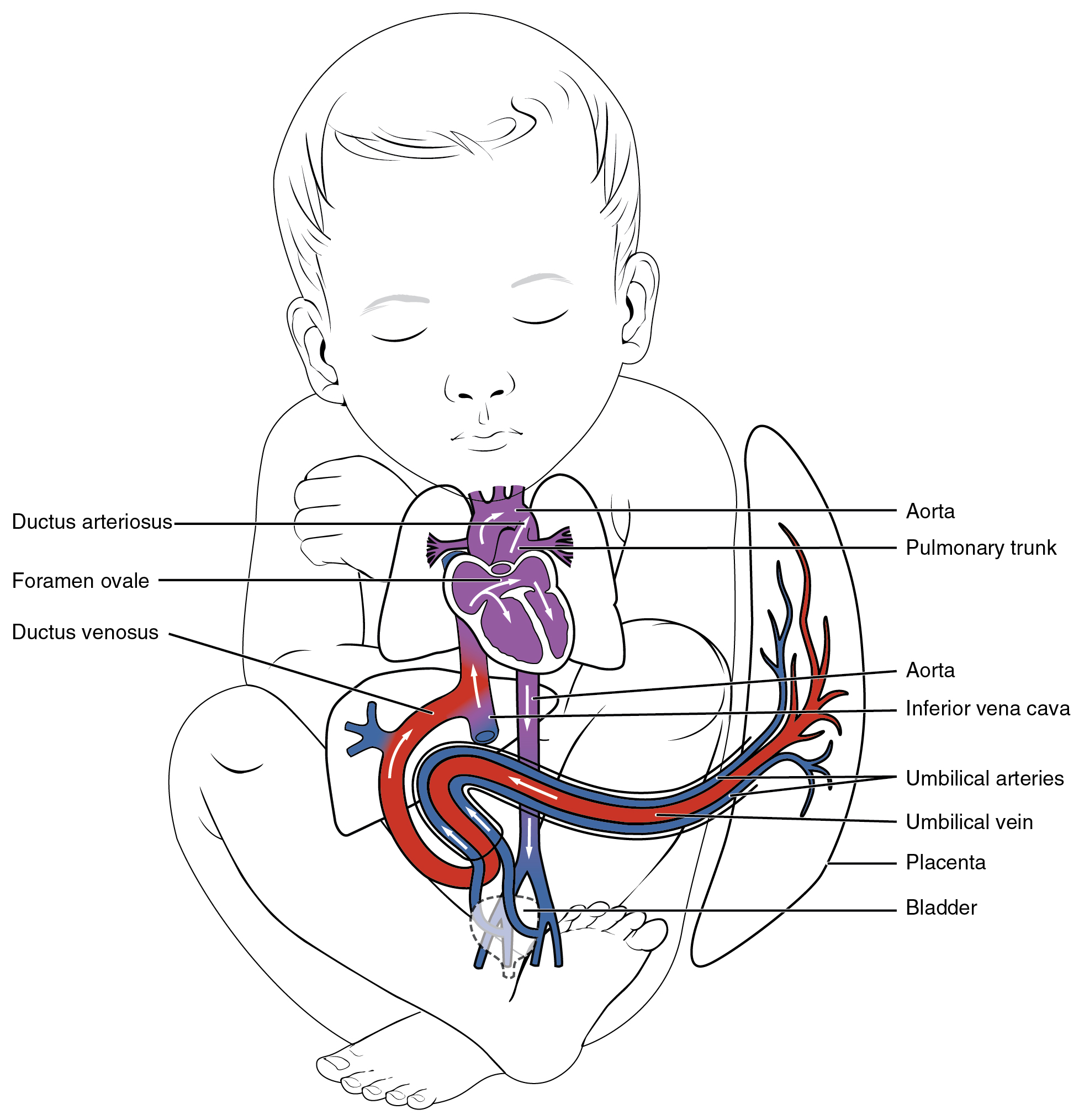
Overview of fetal circulation
Key Concepts
- Fetal circulation
- Adaptation to postnatal life
Overview of Basic Heart Anatomy & Physiology
The heart is primarily composed of muscle, made up of 4 chambers, two upper and two lower, separated by one-way valves.
On the right side the upper chamber, the Right Atrium, receives “used”, or de-oxygenated, blood from the body through the Superior Vena Cava (SVC) and Inferior Vena Cava (IVC). The blood partially runs and partially is pushed into the Right Ventricle through the Tricuspid Valve (TV) during diastole, between contractions. Then, when the heart contracts in systole, the Tricuspid Valve, a one-way valve tethered to the floor of the Right Ventricle, closes and the Pulmonic Valve (PV) is pushed open. The blood is pumped through the Pulmonic Valve and into the Pulmonary Artery, toward the lungs, to be re-oxygenated. When the blood has been reoxygenated it is returned to the Left Atrium through the Pulmonary Veins, where it flows into the Left Ventricle through the Mitral Valve during diastole. When the heart contracts in systole the Mitral Valve, tethered to the floor of the Left Ventricle, closes, and the blood is pushed through the opened Aortic Valve (a small portion of which is seen just to the left of the Mitral Valve) into the Aorta, then on to the body. At the end of the contraction the Aortic Valve closes.
Image Attributions
Comparison of Fetal and Neonatal Circulation from Maternal-Newborn Nursing by Amy Giles, Regina Prusinski, Laura Wallace OpenStax is licenced under CC BY 4.0
End of Image Attribution
Understanding Fetal Circulation
In utero fetal lungs are ‘nonfunctional’ as they are not required for gaseous exchange, as this role is performed by the placenta (Kain & Mannix 2023). In utero the systemic vascular system has high blood flow low resistance, the pulmonary vascular system low blood flow high resistance.
Fetal circulation differs greatly from postnatal circulation as it contains structures that are not required in extrauterine life.
Fetal structures include the:
- Placenta (the conduit between maternal circulation and the fetus enabling oxygen and nutrition to be provided to the fetus as well as removal of waste products from the fetus. NB. maternal and fetal blood do not mix or cross the placenta)
- Ductus venosus (located between the inferior vena cava (IVC) and umbilical vein near the liver, allowing a portion of blood to bypass the liver)
- Ovale foramen (located between the right and left atrium of the heart, allowing a portion of blood to pass through directly from the right atrium to left artium)
- Ductus arteriosus (located between the aortic arch and pulmonary arteries allowing a portion of blood to bypass the nonfunctioning fetal lungs)
- Umbilical vein x 1 (carries oxygenated blood from the placenta to the fetus)
- Umbilical arteries x 2 (carries deoxygenated blood and waste products from the fetus back to the placenta)
(Kain and Mannix 2023, Hillman et al 2012, Marty et al 2023)
Image Attributions
“Fetal Shunts” diagram from Anatomy and Physiology by J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble, Peter DeSaix OpenStax is licenced under CC BY 4.0
End of Image Attribution
Blood Flow in Utero
The umbilical vein carries oxygenated blood from the placenta toward the liver. The ductus venosus is a fetal vessel that connects the umbilical vein to the inferior vena cava, allowing approximately 45-50% of blood to bypass the liver (Kain & Mannix 2023). In the inferior vena cava oxygenated blood carried from the placenta mixes with the deoxygenated blood returning from the fetus’s lower limbs, this mixed blood then enters the right atrium (Kain & Mannix 2023). A large portion of the blood entering the right atrium is shunted across the foramen ovale into the left atrium due to the higher-pressure gradient of the in the left side (Kain & Mannix 2023). It then enters the left ventricle and leaves the heart via the aorta. The blood remaining in the right atrium passes to the right ventricle and leaves the heart via the pulmonary artery. Approximately 10% of this blood enters the lung as the majority flows into the aorta via the ductus arteriosus (Kain & Mannix 2023). As the lungs are not aerated in utero the pulmonary vascular resistance is high. The ductus arteriosus protects the lungs from overloading and allows the right ventricle to strengthen. The placenta has a low resistance, therefore in utero, there is low systemic vascular resistance. (Hillman et al 2012, Marty et al 2023) Via the descending aorta the blood circulates the fetus, and deoxygenated blood then returns to the placenta for re-oxygenation and waste removal via the umbilical arteries (Hillman et al 2012, Marty et al 2023)
Transition to Postnatal Circulation
Major circulatory changes occur at birth. Although most full-term infants achieve this without support, careful nursing/midwifery assessment is required to ensure that infants do make the transition safely.
What happens within the lungs
Transition from fetal to postnatal circulation depends on a number of factors.
These can be divided into:
- Factors that contribute to a postnatal increase systemic vascular resistance (SVR), removal of the placenta, catecholamine surge post birth, & cold extra-uterine environment.
- Factors that contribute to a postnatal decrease pulmonary vascular resistance (PVR), expansion of air-filled lung, establishment of alveolar ventilation and oxygen tension, & successful clearance of fetal lung fluid
At the onset on labour there is a release of catecholamines causing lung fluid production to cease (Hillman et al 2012). Following birth, the fluid in the lungs is replaced with air. Commencement of breathing is dependent on chemical, thermal and sensory stimulation. As the lungs become aerated, there is fall in the pulmonary vascular resistance and an increase in pulmonary blood flow (Hillman et al 2012, Marty et al 2023)
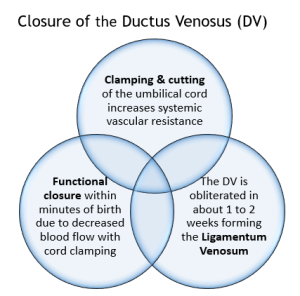
At birth, the umbilical cord is clamped removing the placenta from the circularity system causing a rise in systemic vascular resistance and blood pressure (Bernardo & Dawson as cited in Kain & Mannix 2023). Clamping the umbilical cord stops the flow of blood through the umbilical vein to the ductus venous, enabling blood to flow through the portal system, the umbilical vessels close functionally within minutes (Marty et al 2023), the anatomical remnant of the ductus venosus is the ligamentum venosum (Hillman et al 2012, Marty et al 2023)
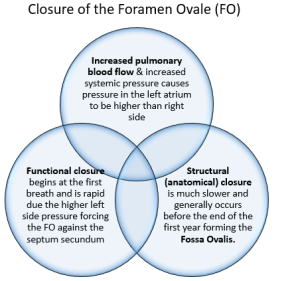
The increased pulmonary blood flow along with increased systemic pressure causes the pressure in the left atrium to be greater than the right atrium, which causes the foramen ovale to functionally close by pressing the valve of the foramen ovale against the septum secundum. This prevents blood flow from the right to the left atrium and ensures blood flow into the right ventricle into the pulmonary trunk. There are two separate forms of foramen ovale closure: functional and structural. functional closure begins at the first breath and is rapid, structural (anatomical) closure is much slower and generally occurs before the end of the first year forming the fossa ovalis (Hillman et al 2012, Marty et al 2023).
Alterations in pressure within the heart also decrease the blood flow across the ductus arteriosus. Closure results from an increase in oxygen (PaO2) levels after birth. During the initial inflation of the lungs a substance called bradykinin is released. Bradykinin is dependent on high PaO2 levels causing smooth muscle to contract. High prostaglandin levels in utero help to maintain duct patency (Hillman et al 2012). With increased blood flow to the lungs an increased amount of prostaglandin is metabolised aiding with the ducts closure. For the first 24-48 hours of life there is often a shunt of blood across the ductus arteriosus from the aorta to the pulmonary trunk. In the healthy term infant ducts are functionally closed by 96 hours. Anatomical closure is much slower occurring by 2–3 weeks after birth (33% of infants), by 2 months (90% of infants) and by 1 year (99% of infants) forming the ligamentum arteriosum (Hillman et al 2012, Marty et al 2023)
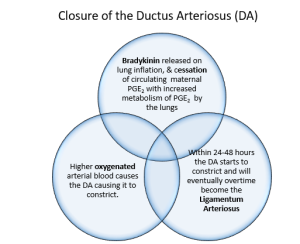
In summary
Several events occur simultaneously during transition to neonatal circulation. Aeration of the lungs with the first breathe leading to decreasing pulmonary vascular resistance (PVR). Removal of the placenta due to the umbilical cord clamping, causes constriction of the ductus venosus and release of catecholamines, which increases systemic vascular resistance (SVR). Closure of the foramen ovale, due increased pulmonary blood flow to the left side of the heart and increasing systemic pressure causes the left atrium pressure to higher than right. Constriction of the ductus arteriosus, due to release of bradykinin in the lungs, the decrease in (PVR), a decrease in circulating maternal prostaglandins (due to the cord being clamped) and a rise in arterial oxygen tension within the lungs
Test your understanding
References
Gardner, S., Carter, B., Enzman-Hines, M. and Niermeyer, S. (2021) Merenstein and Gardner’s Handbook of Neonatal Intensive Care. 9th Edition. Elsevier.
Hillman, N., Kallapur, S., and Jobe, A. (2012). Physiology of Transition from intrauterine to Extrauterine Life. Clinics in Perinatology 39(4) 769-783 https://doi.org/10.1016/j.clp.2012.09.009
Kain, V., and Mannix, T. (2023). Neonatal Care for Nurses and Midwives. Principles for Practice. Second edition. Elsevier.
Marty. M., Kerndt. C. and Lu. F. (2023) Embryology, Fetal Circulation. National Library of Medicine- StatsPearls internet.https://www.ncbi.nlm.nih.gov/books/NBK537149/