Conditions that may impede transition
Key Concepts
- Perinatal asphyxia,
- Hypoxic ischaemic encephalopathy (HIE),
- Patent ductus arteriosus (PDA) and duct dependent/non-duct dependent Congenital Heart Defect (CHD)
Following on from the 5Hs, the following covers some conditions that may impede transition for the newborn infant.
Perinatal Asphyxia
Perinatal Asphyxia refers to a condition resulting from the deprivation of oxygen (hypoxia) to the fetus or the newborn infant (at or around birth) for a duration sufficient to cause discernible harm (Mota-Rojas et al 2022). This condition can lead to significant neurological and systemic complications, highlighting the critical importance of timely intervention and management during the perinatal period (Sinha et al 2017). The term Perinatal covers any period of asphyxia that occurs within five months before birth and up to one month after (Mota-Rojas et al 2022).
Risk factors: Pregnancy-Induced Hypertension, Anaemia, Diabetes, Infection, Substance abuse, Maternal Hypoxemia, Abnormal Uterine Contractions, Cephalopelvic Disproportion, Premature Labor, Prolonged Labor, Multiple Parity, Placenta Previa, Placental Abruption, Placental Insufficiency, Post-Term Pregnancy, Unphysiological Labor, Cord Prolapse, Cord Entanglement, Cord Compression, Congenital Abnormalities, Fetal Anaemia, Iatrogenic Factors, Malpresentation, Prematurity, IUGR
Clinical manifestations:
- Central Nervous System –cerebral oedema, seizures, long-term neurological sequelae, death
- Pulmonary: Persistent Pulmonary Hypertension of the Newborn, Respiratory Distress Syndrome, Meconium Aspiration Syndrome
- Renal: Oliguria, acute renal failure
- Cardiovascular System – tricuspid insufficiency, shock, hypotension
- Metabolic: metabolic acidosis, hypoglycaemia, hypocalcaemia, hyponatremia
- Gastrointestinal – Necrotizing Enterocolitis, hepatic dysfunction
- Hematologic: thrombocytopenia, Disseminated Intravascular Coagulation (DIC)
(Mota-Rojas et al 2022, Gardner et al 2021 Kain and Mannix 2023, Sinha et al 2017)
In summary – Perinatal Asphyxia
In the case of perinatal asphyxia and profound hypoxaemia, the body switches to anaerobic metabolism, leading to increased lactic acid production causing a low pH. This results in a high of carbon dioxide (hypercarbia) which weakens the myocardium, causing a decrease in heart rate and blood pressure. Reduced blood flow to tissues causes further hypoxia, hypercarbia and worsens acidosis (Sinha et al 2017).
Hypoxic Ischaemic Encephalopathy (HIE)
Hypoxic Ischaemic Encephalopathy (HIE) is the clinical manifestation of hypoxic injury to the brain (Mota-Rojas et al 2022). The term used to accurately describe the clinical condition of encephalopathy from asphyxia, without implying the time of brain injury. It occurs because of injury to the brain from a combination of hypoxaemia and decreased cerebral perfusion that leads to ischemia of the brain (Sarnot et al 2020).
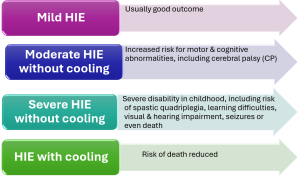
Clinical grading system used to identify severity (Grades 1 – 3) Adapted from Sarnot et al 2020
HIE Grade 1 – MILD (3.9: 1000 births): Hyperalert, spontaneous movement frequent symmetrical, dilated pupils (Mydriasis), tachycardia, weak suck, strong moro, normal muscle tone, posture normal or mild distal flexion. A normal neurologic examination & feeding orally by 2 weeks of age suggest good prognosis (Adapted from Sarnot et al 2020).
HIE Grade 2 – MODERATE (1.1: 1000 births): Lethargic, spontaneous movement decreased, pupils constricted (Miosis), bradycardia, suck weak or absent, weak or absent Moro, muscle tone mild hypertonia, posture mild distal flexion. Symptoms last 2 to 14 days 25% develop cerebral palsy (Adapted from Sarnot et al 2020).
HIE Grade 3 – SEVERE (1: 1000 births): Stuporous, spontaneous movement absent, variable sized pupils, often unequal, poor light responses, absent suck, absent Moro, muscle tone flaccid, posture Intermittent decerebration, fisting, thumb adduction. Lasts hours to weeks poor prognosis (Adapted from Sarnot et al 2020).
Therapeutic cooling (induced hypothermia) has been demonstrated to be an effective neuroprotective strategy (Mota-Rojas et al 2022) and has been implemented in clinical routine for infants that meet the following criteria (exclusion criteria infants birth weight less than 1.8 kg, major congenital malformations, overt bleeding and or imminent death)
- Apgar score of 5 or less at 10 minutes.
- Need for mechanical ventilation or resuscitation at 10 minutes.
- Acidaemia documented in fetal umbilical artery (pH < 7.0 or base deficit ≥ 12 mmol/L);
- Gestation greater or equal to 35 weeks.
- Less than six hours of age
- Evidence of moderate or severe encephalopathy staging, often supported by neuroimaging with evidence of acute brain injury consistent with hypoxia–ischemia
(Mota-Rojas et al 2022, Safer Care Victoria 2023)
HIE without & with therapeutic cooling
A Cochrane systematic review of therapeutic hypothermia in the infant (Jacobs et al., 2013) including eleven randomised controlled trials (RCTs), demonstrated a reduction in mortality with no increase in major disability in those with moderate to severe HIE.
In summary – Hypoxic Ischaemic Encephalopathy (HIE)
HIE is a brain injury caused by hypoxia and reduced blood flow to the brain causing ischaemia. It can result from complications such as perinatal asphyxia. HIE can lead to brain damage, affecting cognitive, motor, and sensory functions, and may result in conditions like cerebral palsy, developmental delays, or intellectual disabilities, depending on the severity of the injury. Treatment focuses on managing symptoms and preventing further damage, early interventions such as therapeutic hypothermia improves neurological outcomes.
Patent Ductus Arteriosus (PDA)
For a quick refresh of the role of the ductus arteriosus refer to the fetal circulation chapter.
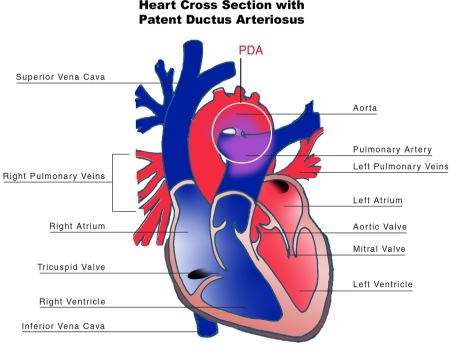
A patent ductus arteriosus (PDA) is a condition where the ductus arteriosus fails to functionally close within approximately 72 hours after birth. The condition leads to abnormal blood flow between the aorta and the pulmonary artery (Dice and Bhatia 2007). The condition is most seen in premature infants, up to 60% of infants less than 28 weeks’ gestation and respiratory distress syndrome (RDS), resulting in a left to right shunt due to a decrease in pulmonary vascular resistance (Mezu-Ndubisi et al 2012), the only blood the lungs require is the blood that supplies the lung tissue at the pulmonary vascular bed (approximately 5-10% of right ventricular output). The ductus diverts the other 90-95% (approximately) of blood into the aorta which goes to the systemic circulation (Kain and Mannix 2023).
Causative factors of a PDA in the premature infant
Factors contributing to the causation of PDA include:
- Development of the contractile tissue in the ductus arteriosus occurs only after 30 weeks’ gestation, therefore oxygen has less ability to vasoconstrict the smooth muscle of the duct.
- The ductus arteriosus remains sensitive to the dilating effects of prostaglandins
- Ventilation, diuretics and stress may trigger an increase in the circulating prostaglandins, relaxing the muscle wall of the ductus. (Mezu-Ndubisi et al 2012).
However, in the term infant a PDA is usually caused by an intrinsic abnormality of the ductal response (PPHN) or a cardiac abnormality (Mezu-Ndubisi et al 2012). In the term infant, with pulmonary hypertension blood shunts right to left through the duct. PPHN can be a result of any condition that causes hypoxia, hypercarbia, acidosis or hypothermia, all these things will induce smooth muscle constriction and when the pressure increases too much blood will take the path of least resistance and pump through the duct arteriosus away from the lungs. (Mezu-Ndubisi et al 2012)
The amount of blood flow through the PDA depends on the size of the duct and the pressure gradient across the duct. This can cause excessive blood flow through the pulmonary circulation into the left atrium and ventricle. This overloading of the left ventricle can result in left ventricle failure and pulmonary oedema with stiff, poorly compliant lungs. Subsequently there is reduced blood flow to the systemic circulation (diastolic steal) (Mezu-Ndubisi et al 2012, Dice & Bhatia 2007). A persistent PDA has been associated with several severe neonatal morbidities, such as: Heart failure, Bronchopulmonary Dysplasia (BPD) due to prolonged respiratory support, Intraventricular haemorrhage (IVH), Renal dysfunction, and Necrotising Enterocolitis (NEC) due to treatment with non-steroidal anti-inflammatories (NSAIDs) (Dice & Bhatia 2007)
*Remember: In- utero the ductus arteriosus remains patent due low arterial oxygen tension and high levels of circulating prostaglandins and normal postnatal adaptation occurs due to constriction of the ductus arteriosus, due to the release of bradykinin within the lungs, a decrease in PVR and a decrease in the circulating prostaglandins and a rise in arterial oxygen tension.
“Patent Ductus Arteriosus” diagram from Wikimedia Commons by NIH is licenced in the Public Domain, CC0
Clinical manifestations of a PDA maybe either symptomatic or asymptomatic these are outlined below:
- Difficult to know whether symptoms are due to L-R shunting or due to the underlying respiratory disease
- Systolic murmur – (sounds like a train)
- Congestive cardiac failure with bounding pulses
- Large deviation between diastolic and systolic BP measurement
- Hypotension
- Breathlessness
- Poor feeding
- Ventilator and oxygen dependency
- Increased vascular markings on chest x-ray
- Heart may be slightly enlarged
- An otherwise asymptomatic PDA may be visualised on ultrasound or systolic murmur may be auscultated – often resolves spontaneously and does not usually require any treatment.
(Mezu-Ndubisi et al 2012, Kain and Mannix 2023).
There are three main strategies utilised by neonatologists when treating an infant with a PDA:
- Conservative management in a hemodynamically stable infant involves moderate fluid restriction and observation.
- Pharmacological management in a hemodynamically unstable infant, there are three pharmacologic treatments are available -indomethacin, ibuprofen lysine (both cyclooxygenase (COX) inhibitors NSAIDs) or acetaminophen/paracetamol (reduces prostaglandin synthesis).
- Surgical ligation is performed if an infant has a hemodynamically significant PDA that results in cardiac dysfunction, renal failure, or respiratory failure.
(Gillam-Krakauer & Mahajan 2023).
In summary – Patent Ductus Arteriosus (PDA)
A PDA is the failure of the normal postnatal adaptation (closure) of the fetal vascular conduit that connects the pulmonary and systemic arterial systems.
- In the preterm infant normal postnatal adaptation may be impacted due to the immature contractile tissue within the ductus arteriosus thus remaining less sensitive to increasing circulation oxygen and increased sensitivity to dilating effects of circulating prostaglandins. In the preterm infant the blood flow through the PDA is left to right increasing the blood flow through the lungs due to the decrease in PVR within the lungs the blood will take the path of least resistance. This results in pulmonary overload and increased work on the left side of the heart due to this increased flow.
- In a term infant a PDA is more commonly seen in the presence of PPHN (increased pulmonary vascular resistance), or infants born with a duct dependent cardiac lesion (forced ductal patency- Prostaglandin E1 (PGE1). In the term infants with pulmonary hypertension blood shunts right to left through the duct, the PVR is higher than the SVR so the blood takes the path of least resistance, this blood is deoxygenated blood so post ductal saturations may be lower (pre ductal blood has not mixed with the de-oxygenated blood yet). The right to left shunt worsens the hypoxia which worsens the vascular constriction, and a viscous cycle ensues.
Congenital heart defects
According to the Victor Chang Cardiac Research Institute (2024), congenital heart defects (CHD) are the most common form of birth defect in Australia, affecting approximately 1 in 100 live-born babies born.
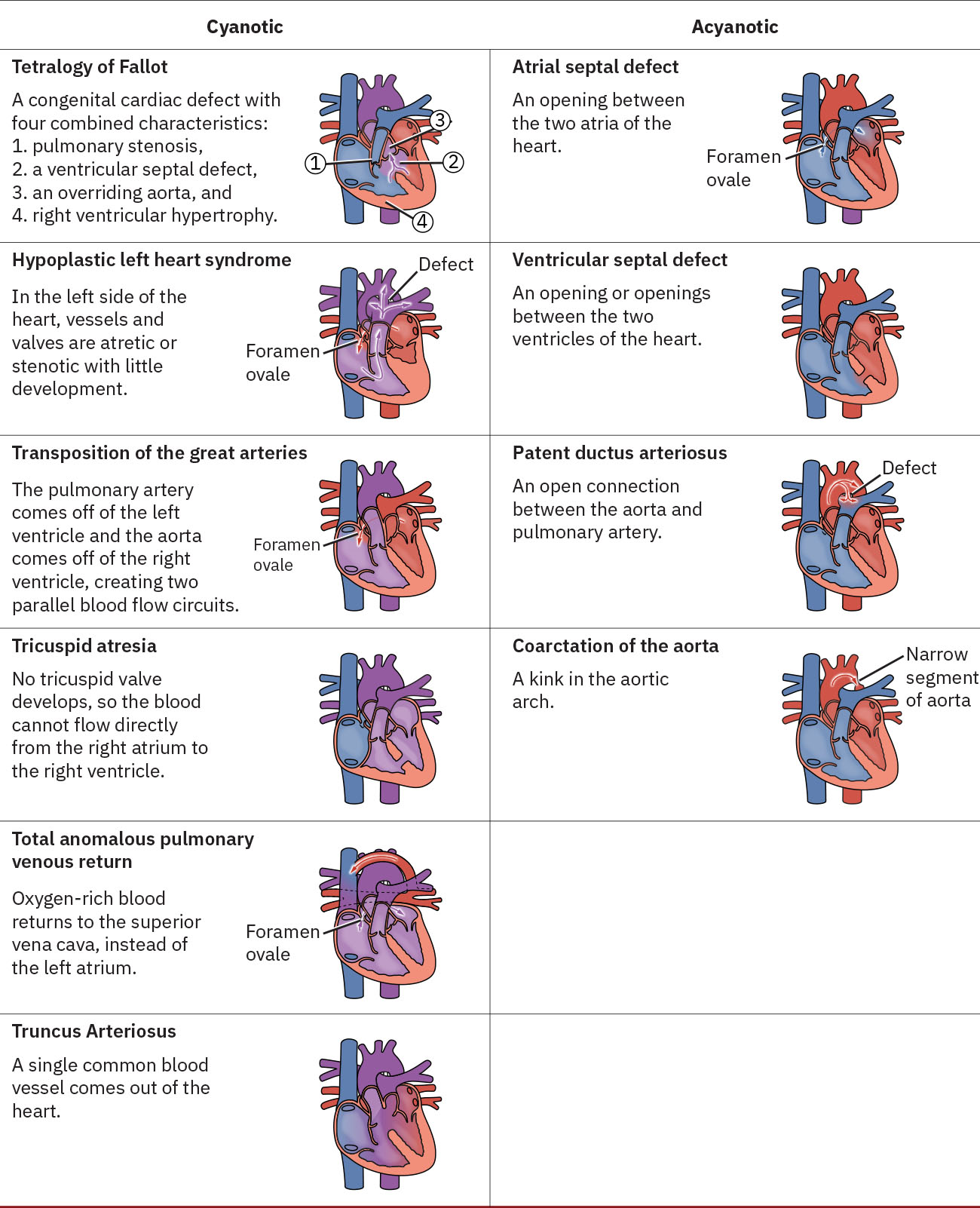
Some CHDs require the duct to remain patent after birth theses are referred to as Duct-dependent congenital heart defects these conditions are reliant on the ductus arteriosus to remain patent after birth to enable adequate blood flow to the body. If diagnosed antenatally these infants will quickly be stabilised, and a prostaglandin infusion commenced to ensure patency of the ductus arteriosus until surgical repair can be undertaken (Kain and Mannix 2023). Supplementary oxygen should be avoided, as oxygen is a known vasoconstrict the smooth muscle of the duct. Some of the duct dependent lesions include: Hypoplastic Left Heart Syndrome (HLHS), Pulmonary Valve Atresia, Tricuspid Atresia, Coarctation of the Aorta, Transposition of the Great Arteries (TGA), Truncus Arteriosus, Total Anomalous Pulmonary Venous Return (TAPVR), Double Outlet Right Ventricle (DORV) (Victor Chang Cardiac Research Institute 2024, The Royal Children’s Hospital accessed 2024 Kain and Mannix 2023).
Image Attribution
Congenital Heart Defects from Maternal-Newborn Nursing by Amy Giles, Regina Prusinski, Laura Wallace OpenStax is licenced under CC BY 4.0
End of Image Attribution
In Summary – Congenital Heart Disease (CHD)
Duct-dependent CHDs in newborn infants rely on the ductus arteriosus to maintain blood flow, and its closure can cause life-threatening circulatory issues. Non-duct-dependent conditions, on the other hand, do not rely on the ductus for circulation but may involve structural heart defects, which affect blood flow without being immediately impacted by the ductus. Both types of conditions require urgent diagnosis and management, often involving surgical intervention or other treatments depending on the severity and nature of the defect.
Test your Understanding
References
Dice, J., and Bhatia. J. (2007). Patent Ductus Arteriosus: An Overview. Journal of Paediatric Pharmacology12(3) pages 138-146 doi: 10.5863/1551-6776-12.3.138
Gillam-Krakauer. M., and Mahajan. K., (2023). Patent Ductus Arteriosus. National Library of Medicine- StatsPearls internet. https://www.ncbi.nlm.nih.gov/books/NBK430758/
Kain, V., and Mannix, T. (2023). Neonatal Care for Nurses and Midwives. Principles for Practice. Second edition. Elsevier.
Mezu-Ndubisi. O., Agarwal. G., Raghavan. A. and et al (2012) Patent Ductus Arteriosus in Premature Infants. Drugs. 7;72(7):907–916. doi: 10.2165/11632870-000000000-00000
Mota-Rojas D, Villanueva-García D, Solimano A, Muns R, Ibarra-Ríos D, Mota-Reyes A. Pathophysiology of Perinatal Asphyxia in Humans and Animal Models. Biomedicines. 2022; 10(2):347. https://doi.org/10.3390/biomedicines10020347
Safer Care Victoria (accessed 2024), Therapeutic hypothermia for hypoxic ischaemic encephalopathy: initiation in special care nurseries. https://www.safercare.vic.gov.au/best-practice-improvement/clinical-guidance/neonatal/therapeutic-hypothermia-for-hypoxic-ischaemic-encephalopathy-initiation-in-special-care-nurseries
Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electrographic study. Arch neurol 1976:33(10);696-705
Sarnat, H., Flores-Sarnat, L., Fajardo, C., Leijser, L., Wusthoff , C., and Mohammad, K. (2020). Sarnat Grading Scale for Neonatal Encephalopathy after 45 Years: An Update Proposal. Pediatric Neurology. Elsevier Inc. Vol 112 pages 75-79 https://doi.org/10.1016/j.pediatrneurol.2020.08.014
The Royal Children’s Hospital. (accessed 2025) Cardiology Fact Sheets. The Royal Children’s Hospital https://www.rch.org.au/cardiology/heart_defects/
Victor Chang Cardiac Research Institute, (2024). Congenital heart disease. Victor Chang Cardiac Research Institute. https://www.victorchang.edu.au/heart-disease/congenital-heart-disease
Hypoxic Ischaemic Encephalopathy
Patent ductus arteriosus
Congenital heart defects