Neonatal Jaundice
Key concepts
- Bilirubin metabolism
- Neonatal Hyperbilirubinemia
- Physiological jaundice
- Pathological jaundice
- Bilirubin toxicity
Neonatal jaundice is a condition in newborns where the skin and eyes appear yellow due to high levels of bilirubin in the blood.
Terminologies
- Jaundice: Is the yellow staining of the skin caused by excessive amounts of bilirubin.
- Bilirubin: Bilirubin in formed from the breakdown of heme containing proteins such as haemoglobin. Haemoglobin is responsible for up to 80% of the bilirubin produced.
- Unconjugated (indirect) bilirubin: Unconjugated bilirubin is the form of bilirubin which has not been bound to albumin and not metabolised by the liver. It is fat soluble and cannot be easily excreted. Also called free bilirubin, when levels become high it deposits in the fatty tissues, such as the skin leading to jaundice and can cross the blood brain barrier leading to kernicterus.
- Conjugated (direct) bilirubin: Once metabolised by the liver bilirubin becomes conjugated and is then water soluble, allowing it to be excreted via the biliary system into the intestines. The conjugated bilirubin is then excreted via stools and urine.
- Physiological jaundice: This is a normal process seen in infants within the first week of life. It occurs during the first few days after birth and is due to a normal physiological process of breaking down of excess red blood cells.
- Pathological jaundice: This refers to jaundice arising from pathological factors that alter the usual process involved in bilirubin metabolism.
- Kernicterus: The yellow staining in the basal ganglia of the brain. It is entirely preventable but is still a risk for all infants with hyperbilirubinemia.
(Moncrieff., 2018, Ullah et al., 2016, Ansong-Assoku et al., 2024, Kain & Mannix 2024, Gardner et al 2021).
Physiology of Bilirubin Metabolism in the newborn
Bilirubin Production:
Bilirubin is a byproduct of haemoglobin breakdown from red blood cells. In newborns, particularly premature infants this process is more active due to increased red blood cell turnover. Since the lifespan of neonatal red blood cells is shorter (approximately 70–90 days) compared to adults (about 120 days), the bilirubin load on the infant’s metabolic system is significantly higher. Newborn infants produce bilirubin at more than twice the adult rate, largely due to relative polycythaemia and accelerated red blood cell breakdown. This elevated bilirubin production, combined with immature liver enzymes responsible for bilirubin conjugation, contributes to the risk of accumulation. Bilirubin levels usually decrease to adult levels within 10 to 14 days after birth. The breakdown of red blood cells occurs in the reticuloendothelial system, primarily the liver and spleen, where aged or damaged red blood cells are removed from circulation.
(Moncrieff., 2018, Ifeanyi Obeagu & Katya, 2022., Ullah et al., 2016, Ansong-Assoku et al, 2024, Kain & Mannix 2024, Gardner et al 2021).
Unconjugated (Indirect) Bilirubin: In the bloodstream, bilirubin initially exists in an unconjugated (indirect) form, which is not water-soluble. This fat-soluble form has an affinity for subcutaneous tissues and the brain, making it potentially harmful and difficult to excrete. To be safely eliminated from the body, it must first be transported to the liver, where it undergoes conjugation. Unconjugated bilirubin is carried through the bloodstream bound to albumin, as it is poorly soluble on its own. Albumin acts as a carrier protein, with one high-affinity binding site and additional lower-affinity sites for bilirubin. When bilirubin is not tightly bound—referred to as “free” bilirubin, it can cross the blood-brain barrier and pose a risk of neurotoxicity. The binding capacity of albumin can be influenced by various factors, including plasma pH, levels of free fatty acids, and certain medications, all of which may reduce its ability to effectively bind bilirubin. (Ifeanyi Obeagu & Katya, 2022., Ansong-Assoku et al, 2024, Kain & Mannix 2024, Gardner et al 2021).
Liver Processing: The liver converts unconjugated (indirect) bilirubin into a water-soluble form known as conjugated (direct) bilirubin by attaching it to glucuronic acid, a process called conjugation. This transformation is catalysed by the enzyme UDP-glucuronosyltransferase (UDPGT), and it requires glucuronic acid, which is synthesized from glucose. In newborn infants, especially during the first few days of life, this conjugation process is less efficient due to immature liver enzyme activity. After unconjugated bilirubin is released into the bloodstream, it quickly binds to albumin and is transported to the liver. Although albumin-bound bilirubin is still considered “free,” it is no longer toxic. Within liver cells (hepatocytes), bilirubin is conjugated with glucuronic acid by the enzyme UDPGT, forming bilirubin glucuronide. This conjugated form is water-soluble, allowing it to be excreted into the bile and eventually reach the intestines for elimination. (Moncrieff., 2018, Ullah et al., 2016, Kain & Mannix 2024, Gardner et al 2021).
Excretion: Once conjugated, bilirubin is excreted into the bile and transported to the intestines. There, it is further broken down into stercobilin and urobilin, which are eventually eliminated in the faeces and urine. However, certain characteristics of the newborn digestive system can interfere with this elimination process. In newborn infants, the gut is initially sterile, and intestinal motility may be slow. These conditions can promote a process known as enterohepatic circulation. Within the intestinal lumen, the enzyme beta-glucuronidase can deconjugate bilirubin—converting it back into its unconjugated form. This unconjugated bilirubin can then be reabsorbed into the bloodstream, increasing the overall bilirubin load in the infant. (Chen & Yuan, 2020; Sisay et al., 2023, Kain & Mannix 2024, Gardner et al 2021).
Image Attribution and text description
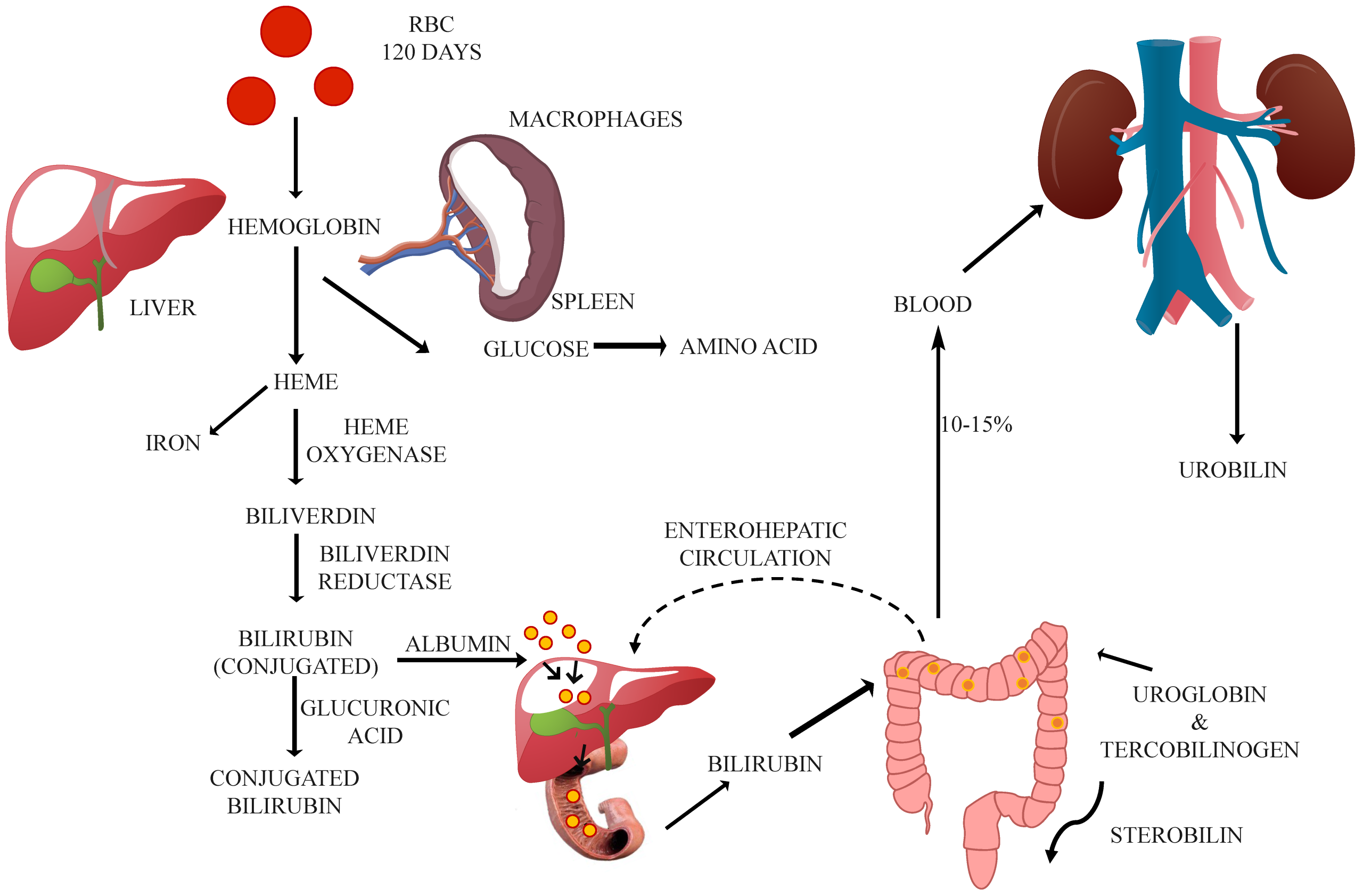
Image is Figure 1 from Hazarika CJ, Borah A, Gogoi P, Ramchiary SS, Daurai B, Gogoi M, Saikia MJ. Development of Non-Invasive Biosensors for Neonatal Jaundice Detection: A Review. Biosensors. 2024; 14(5):254. https://doi.org/10.3390/bios14050254 licenced CC BY 4.0
Image description: Schematic representation that summarizes the complex process of bilirubin metabolism, from the breakdown of heme to the excretion of bilirubin in bile and urine. It also highlights key enzymes like heme oxygenase, biliverdin reductase, and UGT, which are involved in converting bilirubin into its water-soluble form, bilirubin glucuronides. This image also depicts the transport of bilirubin hepatocytes, where further modification occurs before excretion via the bile ducts.
End of Image Attribution and description
Hyperbilirubinemia
Neonatal hyperbilirubinemia is one of the most common clinical disorders in the neonatal period, with 85% of preterm infants and 75% of full-term having visible jaundice (Ansong-Assoku et al, 2024;). Midwives require clinical skills to recognise physiological jaundice, understand the clinical significance and provide the appropriate midwifery management.
Hyperbilirubinemia occurs when serum bilirubin levels have exceeded the normal range for the infant’s gestation, age, and weight. It can be physiological or pathological in nature and can be classified as either increased bilirubin production or decreased bilirubin excretion.
Physiological: As bilirubin clearance transitions from placental to liver-based processing after birth, the newborn liver can easily become overwhelmed. This is especially true in preterm infants, who have insufficient levels of the enzyme UDPGT, typically taking 6 to 7 days to produce adequate amounts. Additional contributing factors include the shorter lifespan of neonatal red blood cells (70–90 days compared to 120 days in adults), increased red blood cell volume, and enhanced enterohepatic circulation, all of which elevate bilirubin production and recycling. Physiological jaundice typically appears between days 2 and 3 of life, peaks around day 6, and resolves by day 10 (Safer Care., 2017, Moncrieff, 2018., Ullah et al., 2016, Hansen & Aslam, 2017, Perrone et al., 2023, Ansong-Assoku et al, 2024, Kain & Mannix 2024, Gardner et al 2021).
However, jaundice is never considered physiological if it:
-
Appears within the first 24 hours of life,
-
Persists beyond 14 days, or
-
Is associated with signs of illness in the infant.
Image Attribution and text description
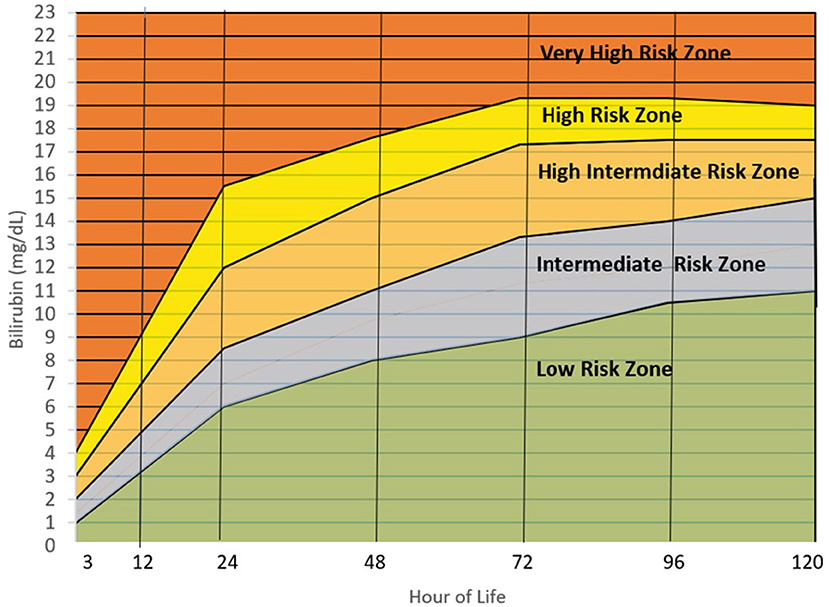
Image is Figure 4 from Villanueva-Uy MET, G. Uy H and Amarillo MLE (2022) Applicability of the hour of life approach in hyperbilirubinemia among Filipino term infants. Front. Pediatr. 10:990919. doi: 10.3389/fped.2022.990919
Image description: The graph shows the concentration of Bilirubin (mg/dL) levels across the first 120 hours of life. The low risk zone rises from 0mg/dL at birth to 11mg/dL over the 120 hours. The intermediate risk zone is at 1mg/dL at birth rising to up to 15mg/dL over the 120 hours. The high intermediate risk zone is at 2mg/dL at birth rising to up to 17.5mg/dL over the 120 hours. The high risk zone is at 3mg/dL at birth rising to up to 19mg/dL over the 120 hours. The very high risk zone is at 4mg/dL at birth rising to over 19mg/dL over the 120 hours.
End of Image Attribution and description
Causes of Physiological Jaundice
Common causes of physiological jaundice include:
- Prematurity: Preterm infant’s red blood cells can last as little as 40 days and this combined with the preterm infant’s slow gut motility, decreased gut bacteria, decreased albumin binding sites and the delayed maturation of glycuronyl transferase significantly increases the preterm infant’s risk of becoming jaundice (
- Medications: Some medications compete with the bilirubin for albumin binding sites, thus making the albumin unavailable for the bilirubin
- Bruising: Bruising, cephalhematoma and swallowed blood can increase the amount of bilirubin due to the rapid breakdown of the red blood cells
- Gastrointestinal system: Inadequate oral intake can cause delayed bacterial colonisation of the gut and increase the entero-hepatic circulation. And or delayed stooling due to the vast amount of bilirubin in meconium, the longer it takes to clear the intestine the greater the amount of bilirubin is absorbed.
- Breastfeeding: Breast milk inhibits glycuronyl transferase and due to the minimal amounts of milk available in the first few days of breastfeeding the entero-hepatic circulation is increased.
(Safer Care., 2017, Moncrieff, 2018., Hansen et al., 2020; Itoh et al., 2022; Sisay et al.,2023, Kain & Mannix 2024, Gardner et al 2021).
Pathological Jaundice
Pathological jaundice is best discussed in relation to time it arises after birth:
- Early: less than 24 hrs Always pathological, usually haemolytic (excess production of bilirubin); ABO incompatibility, RH immunisation, G6PD or sepsis
- Normal: Days two to ten, usually uncomplicated, mild dehydration/insufficient milk supply, haemolysis (breakdown of bruising), polycythaemia (too many RBC, infants of diabetic mother’s, twin to twin transfusion) or infection
- Late: Day fourteen onwards, mostly persistent unconjugated, such as Breast milk jaundice– related to ingredients in breast milk (>fat effects conjugation), haemolysis, hypothyroidism – need thyroxine for removal or late onset sepsis
Causes of Pathological jaundice
Pathological jaundice may appear within the first 24 hours of life, rises rapidly, or persists longer than expected. Causes are grouped by mechanism, Haemolysis (Increased RBC breakdown), Infections, Liver dysfunction (Impaired conjugation), Biliary obstruction (Impaired excretion) or other (bruising, or polycythaemia).
(Safer Care, 2017, Moncrieff, 2018., Ifeanyi Obeagu & Katya, 2022., Ansong-Assoku et al, 2024, Kain & Mannix 2024, Gardner et al 2021).
Common causes of pathological jaundice include (click on the > to expand each disorder):
Bilirubin toxicity
Unconjugated bilirubin is neurotoxic, and its accumulation can lead to acute brain dysfunction known as bilirubin encephalopathy. This condition can progress to kernicterus, a chronic and potentially fatal outcome. Bilirubin encephalopathy refers to the early, acute signs of bilirubin toxicity typically seen in the first few weeks of life, while kernicterus describes the long-term, irreversible neurological damage resulting from such toxicity. There is no clearly defined serum bilirubin level considered entirely safe, as toxicity can occur at varying thresholds. Preterm infants are particularly vulnerable to the harmful effects of bilirubin. Several clinical factors may increase the risk of bilirubin toxicity, including prematurity, the infant’s postnatal age, the rate of bilirubin increase, and medications that reduce bilirubin’s binding to albumin (Ansong-Assoku et al, 2024, Hansen et al., 2020, Kain & Mannix 2024, Gardner et al 2021).
Jaundiced Newborn Receiving Treatment under a Bili Light from Maternal-Newborn Nursing by Amy Giles, Regina Prusinski, Laura Wallace OpenStax is licenced under CC BY 4.0
In Summary
Neonatal hyperbilirubinemia refers to elevated bilirubin levels in newborns and can be physiological or pathological. Physiological jaundice is common, benign, appears around day 3 of life and resolves by day 7-10 and results from increased red blood cell turnover, immature liver function (enzymes), decreased albumin carrying capacity and delayed gastric motility. Pathological jaundice appears within the first 24 hours, rises rapidly, or lasts longer than expected, and may be due to haemolysis (e.g., ABO/Rh incompatibility), infections, metabolic disorders, or biliary obstruction. Pathological causes often require urgent evaluation and treatment to prevent complications like kernicterus.
Test your understanding
References
Ansong-Assoku, B., & Ankola, P. A. (2023). Neonatal Jaundice. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK532930/
Chen, K., & Yuan, T. (2020). The role of microbiota in neonatal hyperbilirubinemia. American Journal of Translational Research, 12(11), 7459–7474.
Gardner, S., Carter, B., Enzman-Hines, M. and Niermeyer, S. (2021) Merenstein and Gardner’s Handbook of Neonatal Intensive Care. 9th Edition. Elsevier.
Hansen, T. W. R., Wong, R. J., & Stevenson, D. K. (2020). Molecular Physiology and Pathophysiology of Bilirubin Handling by the Blood, Liver, Intestine, and Brain in the Newborn. Physiological Reviews, 100(3), 1291–1346. https://doi.org/10.1152/physrev.00004.2019
Itoh, S., Okada, H., Koyano, K., Nakamura, S., Konishi, Y., Iwase, T., & Kusaka, T. (2023). Fetal and neonatal bilirubin metabolism. Frontiers in Pediatrics, 10, 1002408. https://doi.org/10.3389/fped.2022.1002408
Ifeanyi Obeagu, E., & Charlene Katya, M. (2022). A Systematic Review on Physiological Jaundice: Diagnosis and Management of the affected Neonates. Madonna University Journal of Medicine and Health Sciences; Madonna University Press. https://journal.madonnauniversity.edu.ng/index.php/medicine/article/view/72/79
Kain, V., and Mannix, T. (2023). Neonatal Care for Nurses and Midwives. Principles for Practice. Second edition. Elsevier.
Moncrieff, G. (2018). Bilirubin in the newborn: Physiology and Pathophysiology. British Journal of Midwifery. https://www.britishjournalofmidwifery.com/content/clinicalpractice/bilirubin–in–the–newborn–physiology–and–pathophysiology/
Safer Care Victoria. (2017). Jaundice in Neonates. Victoria State Government. https://www.safercare.vic.gov.au/best–practice–improvement/clinicalguidance/neonatal/jaundice–in–neonates (accessed November 2024)
Sisay, B. D., Abebe, R. F., Kassie, A. A., Wondimu, M. G., & Kassie, G. A. (2023). Determinants of neonatal jaundice among neonates admitted to neonatal intensive care unit in public hospitals of Sidama Region, Sidama, Ethiopia, 2022: an unmatched case-control study. The Pan African Medical Journal, 45, 117. https://doi.org/10.11604/pamj.2023.45.117.40472
Ullah, S., Rahman, K., & Hedayti, M. (2016). Hyperbilirubinemia in Neonates: Types, Causes, Clinical Examinations, Preventive Measures and Treatments: A Narrative Review Article. Iran J Public Heath, 45(5) 558-568. PMC4935699
Red blood cells