Neonatal hyperglycaemia is commonly caused by prematurity, stress, sepsis, or excessive glucose administration, and can lead to complications such as dehydration, electrolyte imbalance, increased risk of intraventricular haemorrhage, and worsened outcomes in haemodynamically unstable infants.
Glucose Homeostasis
Key Concepts
- Glucose homeostasis in utero and after birth
- Neonatal hypoglycaemia in the premature and infant of a diabetic mother
- Neonatal hyperglycaemia
Glucose homeostasis requires a complex balance between glucose utilisation and glucose production and intake. This balance is controlled by insulin and various other hormones that regulate the metabolic pathways for fuel production (i.e. glycogenesis, glycogenolysis, gluconeogenesis, lipolysis and ketogenesis). Disturbances of these metabolic and endocrine processes may occur in infants due to developmental immaturity (Amendoeira et al 2020).
Terminologies
- Glycogenesis: the process by which glucose not required by the body for immediate energy needs is converted to glycogen and stored in the liver, heart and skeletal muscles.
- Glycogenolysis: the process by which glycogen is released from the liver during fasting.
- Gluconeogenesis: the production of glucose in the liver by means of non-glucose precursors such as lactate, pyruvate, glycerol and amino acids.
(Amendoeira et al 2020)
Glucose Production and Metabolism
Glucose can be metabolized in the body in several ways:
- Production of energy
- Storage as glycogen
- Conversion to gluconeogenic precursors
Fetal Glucose Homeostasis in utero
The fetus receives a continual supply of glucose from its mother via the placenta necessary for growth, at 60-70% of maternal value. Glucose is transported across the placenta by facilitated diffusion, but during maternal starvation or placental insufficiency, the fetus is capable of endogenous glucose production. The fetal diet is high in carbohydrates and low in fat. The brain, red blood cells (RBC’s) and renal medulla are dependent on glucose for energy. The fetus can regulate its glucose concentrations independently of maternal hormones, as it produces its own insulin, cortisol, growth hormone and thyroxin. This can be seen in cases of placental insufficiency when gluconeogenesis is activated due to fetal hypoglycaemia, and in the fetus of the diabetic mother who responds to the high placental transfer of glucose by secreting high concentrations of insulin. Under extreme circumstances, fetal blood glucose control can fail. When placental insufficiency leads to intrauterine growth restriction (IUGR), fetal hypoglycaemia often occurs. Profound and prolonged fetal hypoglycaemia has been linked with long term neurological damage (RCH 2023, Amendoeira et al 2020, Gardner et al 2021, Kain and Mannix 2023, Hume et al 2005).
Neonatal Glucose Homeostasis after birth
At the time of birth, the infant must rapidly switch from the continuous glucose supply from the placenta to maintaining and regulating its own supply of glucose. The infant’s blood glucose level falls reaching a nadir (lowest point) at 1 to 2 hours of age (Rozance 2024, Safer Care 2018); however, the depth and speed of postnatal drop depends on prebirth insulin levels. In the first few hours of life, the neonatal brain metabolises lactate so that even if the glucose concentration is low, the brain will not be fuel deficient (Amendoeira et al 2020). The infant gradually mobilizes glucose to meet energy needs by secreting glucagon and catecholamine and suppressing insulin release. Hepatic glycogen is rapidly depleted if feeding is not established early (Amendoeira et al 2020, Gardner et al 2021, Kain and Mannix 2023). Thus, even if a healthy term newborn is not fed soon after birth, blood glucose levels rise at 3 to 4 hours of age. (Safer Care 2018, RCH 2023).
The below approximates the rate of glucose oxidation which is proportional to brain to body mass ratio:
Preterm infant: 3-15mg/kg/min
Term infant: 5-15mg/kg/min
Adult: 2-5mg/kg/min
(Rizzo et al 2022, Elhassan and Kaiser 2011)
In Summary – Glucose homeostasis
Glucose homeostasis in the neonate is the process of maintaining stable blood sugar levels greater than 2.6mmol/l. With the clamping of the umbilical cord, at birth the maternal glucose supply ceases, and the infant then relies on stored glycogen, gluconeogenesis, and hormonal regulation to produce glucose. Hormones like glucagon and cortisol help raise glucose levels, while insulin decreases. This process is vital for brain function, and failure to maintain glucose levels, especially in premature or hemodynamically unwell infants can lead to serious complications like hypoglycaemia, seizures, brain injury, and development delays.
Pathophysiology of Hypoglycaemia
Hypoglycaemia occurs in 5-15% of all neonates and is one of the most common metabolic disorders. If untreated hypoglycaemia has a high mortality rate, with prolonged or severe neonatal hypoglycaemia resulting in brain injury or adverse neurological outcomes (RCH 2023). The immediate postnatal drop in the infant’s blood glucose level is a normal physiologic response. It is the failure of blood glucose levels to rise after the first few hours which is considered a pathological response. Hypoglycaemia is usually a result of inadequate hepatic glucose production that cannot meet peripheral demand (Amendoeira et al 2020, Kain and Mannix 2023). Hypoglycaemia is defined as the lowest level of blood glucose and the longest time it can be tolerated by the infant without causing cerebral injury. With most organisations, the lowest accepted blood glucose level is 2.6mmol/L (Amendoeira et al 2020, Gardner et al 2021, Kain and Mannix 2023, Hume et al 2005).
During hypoglycaemia, the brain increases blood flow to improve glucose delivery and uses alternative fuels if they are available (Amendoeira et al 2020). Unfortunately, preterm infants and those who are small for gestational age are severely limited in the ability to mount a counter regulatory response, so these infants are dependent on receiving an adequate glucose supply (Amendoeira et al 2020, Gardner et al 2021, Kain and Mannix 2023, Hume et al 2005). Prolonged hypoglycaemia causes biological changes at the cell level that can damage the neuronal and glial cells of the brain. It is the accumulation of amino acids during hypoglycaemia that leads to cellular depolarization, impairing neuronal growth and cellular death (Amendoeira et al 2020). A hypoglycaemia infant may be asymptomatic or display nonspecific signs that can often by associated to other clinical conditions. Clinical manifestations may include tremors or jitteriness, high pitched or weak cry, respiratory distress (apnoea, irregular respirations, tachypnoea and cyanosis), hypotonia, lethargy, irritability, feeding difficulties, vomiting, seizures, coma, or even death (Amendoeira et al 2020).
Hypoglycaemia can be caused by the following conditions (Click on the condition for more information):
(Amendoeira et al 2020, Gardner et al 2021, Kain and Mannix 2023, Hume et al 2005).
Diagnosis
As our therapeutic goal is prevention of hypoglycaemia, when infants are susceptible to low blood sugar levels prompt glucose screening should occur and or may be admitted to a special care nursery for close monitoring. Screening for hypoglycaemia can be performed as follows:
-
- Blood reagent strip- these can be inaccurate at the lower end of the range tending to under read the true blood glucose.
- True blood glucose- performed on the blood gas machine with a capillary blood tube.
- Laboratory serum glucose test- for all extremely low readings
Complications
Complications can include:
-
- The normal physiologic drop in neonatal blood sugar after delivery is not considered to have any clinical significance.
- There are no human studies that have addressed what duration of hypoglycaemia is harmful, however the literature suggests that prolonged periods (12 to 24 hours) in the at-risk population may lead to neurological sequelae (Abramowski et al 2023)
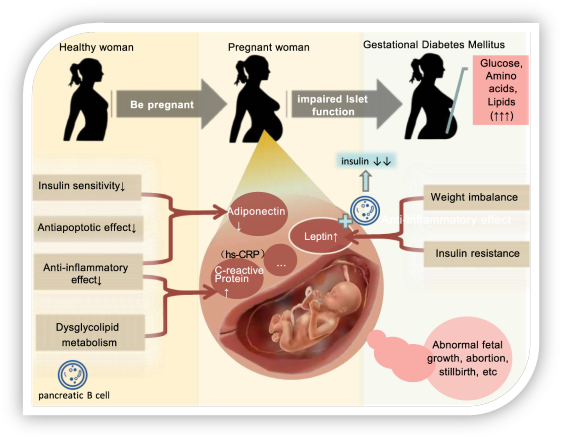
The Infant of a diabetic mother- fetal hyperinsulinemia
Maternal hyperglycaemia can result in fetal hyperglycaemia and fetal hyperinsulinemia. Insulin is the main ‘growth hormone’ of the fetus and therefore infants of a poorly controlled diabetic mothers (IDM) are often macrosomic (>4000g) or large for gestational age (>90th percentile).
The main problem is that at birth the transplacental glucose supply which the fetus has received all throughout development is abruptly stopped. It is because the fetus has developed high concentrations of insulin, blood glucose levels drop.
“Mechanisms of gestational diabetes mellitus” by Yang Liu, Dan Yang Li, Alayi Bolatai and Na Wu licensed under CC BY-NC 3.0
Effects of maternal hyperglycaemia
Fetal effects of maternal hyperglycaemia: Poor glycaemic control during embryo development can result in an increase in congenital malformations including cardiac defects, central nervous system defects (i.e. anencephaly, spina bifida), genitourinary and limb defects. These are not seen in mothers who develop gestational diabetes after the first trimester (Sinha 2017)
Peripartum risks/complications caused by fetal hypoxia during maternal hyperglycaemia which can lead to a substantial increase in adrenal catecholamines causing: Fetal hypertension, cardiac hypertrophy, stimulation of erythropoietin, leading to polycythaemia and blood hyperviscosity, increased risk of thrombus, hyperbilirubinemia (due to increased red cell mass) (Sinha 2017)
Intrapartum risks/complications of the macrocosmic fetus caused by hyperinsulinemia: Shoulder dystocia, clavicular fractures, brachial plexus injury, facial nerve injury, cephalhematoma, asphyxia, perinatal and neonatal mortality (Sinha 2017)
Postnatal neonatal risks/complications of poorly controlled maternal diabetes during pregnancy:
- Polycythaemia and hyperviscosity due to increased erythropoiesis secondary to fetal arterial hypoxaemia secondary to hyperinsulinemia.
- Hypoglycaemia, Hypocalcaemia due to functional hypoparathyroidism and hypomagnesemia. This occurs in approximately 50% of insulin dependent diabetics. Clinical presentation includes irritability, tremors, jitters, tongue thrusting, twitches, apnoea or seizures.
- Hypomagnesaemia due to maternal hypomagnesaemia and increased renal losses with glycosuria.
- Hyperbilirubinemia due to polycythaemia, increased extravascular haemolysis from bruising or cephalhematoma, delayed oral feeding causing increased enterohepatic circulation and liver immaturity.
- Hypertrophic congestive cardiomyopathy which is usually asymptomatic and resolves by 8 to 12 weeks.
- Respiratory distress due to delayed fetal maturation (insulin impedes glucocorticoid effect), prematurity, increased incidence of caesarean section in near term deliveries which can be complication by transient tachypnoea of the newborn (‘wet lung’)
(Sinha 2017)
In Summary: Neonatal Hypoglycaemia
Premature Infant: these infants are at high risk of hypoglycaemia due to limited glycogen and fat stores, immature liver function, and underdeveloped metabolic pathways needed for glucose production and metabolism.
Infant with Hypothermia and Respiratory Distress: These infants use more glucose and oxygen to generate heat and meet increased energy demands; this rapidly depletes glucose stores, leading to hypoglycaemia and can exacerbate hypoxia in an already compromised infant.
Infant of a Diabetic Mother (IDM) with Hyperinsulinemia: These infants produce higher levels of insulin in-utero in response to high maternal glucose load during pregnancy, the high insulin production continues after birth despite the maternal supply of glucose stopping, the infant is reliant on its own glucose stores which are limited until feeding established.
Neonatal Hyperglycaemia
Neonatal Hyperglycaemia in the infant, is defined as a blood glucose level greater than 8 mmol/l and is seen less often than neonatal hypoglycaemia. It is usually the result of a high glucose production or infusion rate or a low glucose uptake rate. The prevalence of neonatal hyperglycaemia appears to be increasing in parallel with the increased survival of extremely low birth weight infants and the early use parental nutrition in these infants.
Hyperglycaemia in the infant is a transient disease which resolves spontaneously and can occur due to premature infants receiving intravenous solutions containing glucose may not have the ability to adapt to the exogenous administration of glucose by suppressing endogenous glucose production. There may also be an ineffective insulin response which leads to a general insensitivity to glucose. Certain medications, including caffeine or steroids may cause an increase in blood glucose as well as stressors such as surgery, pain or sepsis may increase circulating catecholamine, which will result in hyperglycaemia.
Clinical Presentation: Most infants are asymptomatic. There may display glycosuria, and osmotic diuresis may cause fluid and electrolyte imbalances with dehydration. Infants, unlike adults, do not develop ketosis or metabolic acidosis.
Diagnosis
As for hypoglycaemia, hyperglycaemia can be detected by using a blood reagent strip. Such strips are more reliable for high blood glucose levels, however, still may provide an inaccurate result of up to 0.5 to 1.0 mmol/l. High blood sugar levels should be confirmed by a true blood glucose test performed in the lab or using the blood gas machine. It is also recommended to monitor urine for glycosuria. It is important to note that certain infants, particularly preterm, have a low renal threshold for glucose excretion, so for these infants, glycosuria may be present in normoglycaemia.
Management
The first step in management is to find and treat any underlying condition that is causing the hyperglycaemia. In many cases decreasing the amount of intravenous fluid solution administered to the infant is adequate to correct hyperglycaemia. Further from this the dextosity of the intravenous solution can be reduced. Lipid infusions are also known to cause an increase in serum glucose levels; therefore, a reduction or cessation of lipids may be required. In the case of severe hyperglycaemia, insulin therapy may be required. There is controversy regarding the blood glucose level at which insulin therapy should be commenced. One side of this controversy is in favour of early introduction of insulin which allows the infant to tolerate higher glucose loads which will help to provide calories for growth.
Complications
Hyperglycaemia can cause an osmotic diuresis, which draws fluid from the intracellular to the extracellular space, leading to dehydration. These changes in blood osmolarity and fluid shifts may be a risk factor for intraventricular haemorrhages and cerebral damage.
In Summary – Neonatal Hyperglycaemia
Test your understanding
Image Attributions
A baby in hospital by Alana Souza from Pexels
Photo of a Woman Carrying Her Newborn Baby by Craig Adderley from Pexels
Baby Wrapped in White Cloth by Johnathan Borba from Pexels
A baby in a crib by Olivia Anne Snyder used under Unsplash licence
A woman is holding a baby while another person is holding a syringe by Johnathan Borba from Pexels
End of Image Attributions
References
Amendoeira, S., McNair, C., Saini, J., and Habib, S. (2020) Glucose Homeostasis and the neonatal brain: A sweet relationship. Neonatal Network. Vol 33 (3). http://dx.doi.org/10.1891/0730-0832.39.3.137
Abramowski, A., Ward, R., and Hamdan, A.(2023) Neonatal Hypoglycemia. National Library of Medicine- StatPearls (internet). . https://www.ncbi.nlm.nih.gov/books/NBK537105/
Elhassan, N., and Kaiser, J. (2011). Parenteral Nutrition in the Neonatal Intensive Care Unit. NeoReviews; Vol. 12(3). https://doi.org/10.1542/neo.12-3-e130
Gardner, S., Carter, B., Enzman-Hines, M. and Niermeyer, S. (2021) Merenstein and Gardner’s Handbook of Neonatal Intensive Care. 9th Edition. Elsevier.
Hume, R., Burchell, A., Williams, F., and Kah, D. (2005). Glucose Homeostasis in the newborn. Early Human Development. Vol 81(1), Pages 95-101 https://doi.org/10.1016/j.earlhumdev.2004.10.005
Kain, V., and Mannix, T. (2023). Neonatal Care for Nurses and Midwives. Principles for Practice. 2nd Edition. Elsevier.
Rozance, P. (2024) Management and outcome of neonatal hypoglycemia. UpToDate https://www.uptodate.com/contents/management-and-outcome-of-neonatal-hypoglycemia/print#:~:text=During%20the%20normal%20transition%20to,of%20approximately%2055%20mg%2FdL. (accessed November 2024)
Safer Care Victoria (2018). Hypoglycaemia in neonates. SaferCare Victoria. https://www.safercare.vic.gov.au/best-practice-improvement/clinical-guidance/neonatal/hypoglycaemia-in-neonates (accessed November 2024)
Sinha, M., Miall, L., and Jardine, L. (2017) Essential Neonatal Medicine. Sixth edition. Wiley and sons
The Royal Children’s Hospital [RCH] (2023). Neonatal hypoglycaemia. The Royal Children’s Hospital https://www.rch.org.au/rchcpg/hospital_clinical_guideline_index/Neonatal_hypoglycaemia/ (Accessed November 2024)
Rizzo, V., Capozza, M., Panza, R., Laforgia, N., and Baldassarre, M. (2022) Macronutrients and Micronutrients in Parenteral Nutrition for Preterm Newborns: A Narrative Review. Nutrients 6;14(7):1530. doi: 10.3390/nu14071530
definition
Intrauterine growth restriction